Anakinra Corona - Coronavirus Anakinra A Promising Treatment Sortiraparis Com
Anakinra a recombinant interleukin-1 receptor antagonist is known to be effective in several hyperinflammatory diseases. EMA has started evaluating an application to extend the use of Kineret anakinra to include treatment of coronavirus disease 2019 COVID-19 in adult patients with pneumonia who are at risk of developing severe respiratory failure inability of the lungs to work properly.
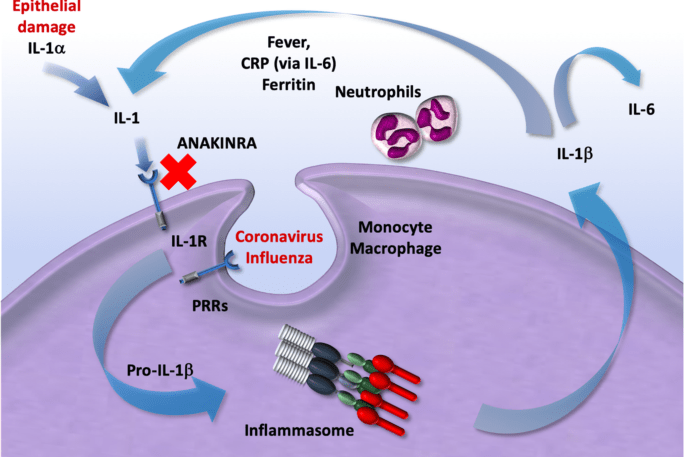
Anakinra ANA is a recombinant human decoy IL-1Ra and therefore blocks IL-1α and IL-1β.
Anakinra corona. Immunglobulinen Dosierungsschema su und Anti IL-1 Anakinra erwogen werden. Impfstoff-Nebenwirkung ADEEin Schreckgespenst für die Corona-Impfung. Ruxolitinib ist derzeit nicht etabliert sollte möglichst im Rahmen von kontrollierten Studien erfolgen und bleibt eine Einzelfallentscheidung.
However anakinra might be a safer alternative to dexamethasone when considering the risk of secondary infections which was low in early reports but might rise after more widespread use of dexamethasone. The treatment involves using a patients own T cells a type. In adults and children with sHLH triggered by SARS-CoV-2 or a similar coronavirus what is the clinical effectiveness of anakinra compared with supportive treatment.
Important considerations for clinical trials. Some new studies have considered intravenous anakinra for related conditions including hyperinflammation in people with COVID-19 and ARDS. New YorkMailand Der Zytokinsturm Cytokine-release syndrome CRS zu dem es.
We report the first successful treatment with the IL-1 receptor antagonist anakinra in association with the most promising and available antiviral therapy of a severe case of novel coronavirus disease 2019 COVID-19. Therefore no evidence is available to determine whether anakinra is effective safe or cost effective for treating adults and children with sHLH triggered by SARS-CoV-2 or a similar coronavirus. Anakinra ist ein rekombinant hergestellter humaner Interleukin-1-Rezeptorantagonist aus der Gruppe der Immunsuppressiva.
Er gleicht dem nativen humanen IL-1-Rezeptorantagonisten mit Ausnahme der zusätzlichen Aminosäure Methionin am N-Terminus und. We investigated the effects of anakinra on inflammatory parameters and clinical outcomes in critically ill mechanically ventilated COVID-19 patients with clinical features of hyperinflammation. 44 45 An additional benefit might be seen in specific populations such as patients with diabetes who are more susceptible to secondary infections and less likely to tolerate hyperglycaemia.
Mailand Eine hoch-dosierte intravenöse Behandlung mit dem Interleukin-1-Rezeptor-Antagonisten Anakinra hat in einer Behandlungsserie in Lancet Rheumatology. Relevant trials were identified by searching literature until 24 April 2021 using the following terms. We describe the diagnosis clinical course and management of the case includin.
The overall objective of the study is to determine the therapeutic effect and tolerance of Anakinra in patients with moderate severe pneumonia or critical pneumonia associated with Coronavirus disease 2019 COVID-19. Eine antiinflammatorische Therapie mit Anti IL-6 Anti IL-1 bzw. Rheumamittel Anakinra könnte Neurotoxizität verhindern.
Anakinra in COVID-19. Der Wirkstoff wird gentechnologisch aus Escherichia coli-Stämmen isoliert und setzt sich aus 153 Aminosäuren zusammen. In stage 1 an estimated 80 to 84 of infected patients are slightly symptomatic.
Selection criteria Trials evaluating the effect of anakinra on the need for invasive mechanical ventilation and mortality in hospitalized non-intubated patients with COVID-19. The research focused on an Italian hospital that treated a number of coronavirus patients with ARDS in mid-March. Anakinra interleukin 1 coronavirus COVID-19 SARS-CoV-2.
It has been postulated that anakinra a recombinant IL-1 receptor antagonist might help to neutralise the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2-related hyperinflammatory state which is considered to be one cause of acute respiratory distress among patients with COVID-19. The COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 has caused more than 320 000 deaths as of May 19 2020. COVID-19 deaths are primarily caused by acute respiratory distress syndrome ARDS and by a cytokine storm syndromeie a.
A new study published in the medical journal The Lancet Rheumatology adds to the evidence that this drug may be worth a try in certain coronavirus patients. Einer der am weitesten entwickelten Covid-19. A drug like anakinra however might.
Hier für einen Antikörpertest im Labor. Der Erreger der aktuellen Pandemie erhielt diesen Namen im. Die Erreger von SARS in den Jahren 20022003 MERS und mehreren Erkältungsformen zählen ebenfalls dazu.
Coronavirus disease 2019 COVID-19 the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2associated disease can be classified into three clinical stages. Es handelt sich um den Oberbegriff für eine Familie von Viren die Menschen oder Tiere befallen. Supportive care may involve treatment with corticosteroids IVIG etoposide organ support ventilation renal replacement therapy transfusions etc and antimicrobials.
Early increase of soluble urokinase plasminogen activator receptor suPAR serum levels is indicative of increased risk of progression of coronavirus disease 2019 COVID-19 to respiratory failure. Full Title A Phase II Study of IL-1 Receptor Antagonist Anakinra to Prevent Severe Neurotoxicity and Cytokine Release Syndrome in Patients Receiving CD19-Specific Chimeric Antigen Receptor CAR T Cells And to Treat Systemic Inflammation Associated with COVID-19 Purpose CAR T-cell treatment is a type of immunotherapy.

Anakinra In Hospitalized Patients With Severe Covid 19 Pneumonia Requiring Oxygen Therapy Results Of A Prospective Open Label Interventional Study International Journal Of Infectious Diseases

Immunsuppressivum Zulassungsantrag Fur Anakinra Bei Covid 19 Pz Pharmazeutische Zeitung

Novel Paediatric Presentation Of Covid 19 With Ards And Cytokine Storm Syndrome Without Respiratory Symptoms The Lancet Rheumatology

Greek Study Suggests Mortality Benefit For Kineret In Covid 19 2021 05 20 Bioworld
Plos One Glucocorticoids With Low Dose Anti Il1 Anakinra Rescue In Severe Non Icu Covid 19 Infection A Cohort Study

Il 1 Receptor Antagonist Anakinra In The Treatment Of Covid 19 Acute Respiratory Distress Syndrome A Retrospective Observational Study The Journal Of Immunology

Anakinra For Severe Forms Of Covid 19 A Cohort Study The Lancet Rheumatology

Covid 19 The Role Of Immunomodulators In Treatment
Biocentury A Mechanistic View Of Four Compounds That Disrupt Interleukin Pathways To Treat Severe Covid 19

Interleukin 1 Blockade With High Dose Anakinra In Patients With Covid 19 Acute Respiratory Distress Syndrome And Hyperinflammation A Retrospective Cohort Study The Lancet Rheumatology
Biocentury A Mechanistic View Of Four Compounds That Disrupt Interleukin Pathways To Treat Severe Covid 19

Interleukin 1 Receptor Antagonist Anakinra In Association With Remdesivir In Severe Covid 19 A Case Report International Journal Of Infectious Diseases

Anakinra Lowers Ventilation Mortality Risk In Non Intubated Patients With Covid 19

Abschluss Der Anakinra Studie Charite
Coronavirus Anakinra A Promising Treatment Sortiraparis Com

Anakinra Reduces Ventilation Need Mortality In Covid 19

Covid 19 Rheumamittel Anakinra Erzielt In Beobachtungsstudie

Favorable Anakinra Responses In Severe Covid 19 Patients With Secondary Hemophagocytic Lymphohistiocytosis Sciencedirect

Study Backs Cytokine Targeting For Covid 19 Tx Medpage Today


