Influenza Vaccine Production | Egg Based Pilot Influenza Vaccine Production Process Download Scientific Diagram
The four different compositions of influenza vaccine differ in antigen components and structural organization. These projections may change as the season progresses.
Better Influenza Vaccines An Industry Perspective Journal Of Biomedical Science Full Text
Egg-based production is the most commonly used technique for manufacturing of influenza vaccine.

Influenza vaccine production. For the 2021-2022 season manufacturers have projected they will provide as many as 188 to 1200 million doses of influenza vaccine for the US. Sanofi Pasteur the vaccines division of Sanofi EURONEXT. Historical Reference of Vaccine Doses Distributed A ten year look at influenza vaccine distribution in the US.
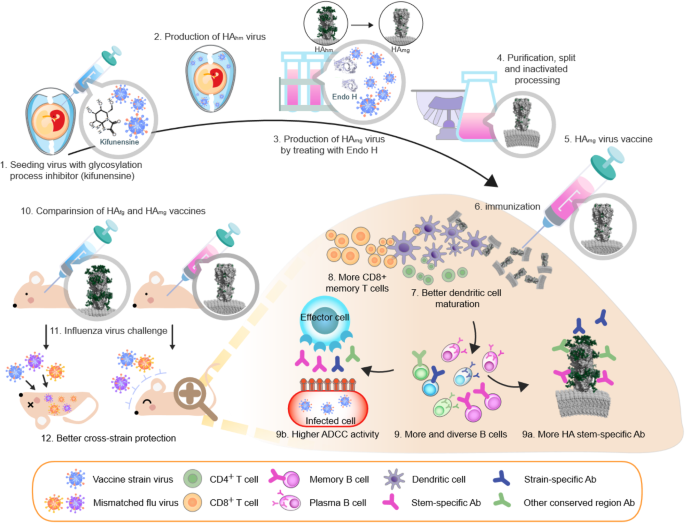
Vaccines are currently produced by gene techniques ie. Although most influenza vaccines are produced using egg proteins they are still recommended as safe for people who have severe egg allergies as no increased risk of allergic reaction to the egg-based vaccines has been shown for people with egg allergies. Here we evaluate the potential of whole inactivated vaccines based on chemical and physical methods as well as new approaches to generate cross-protective immune responses.
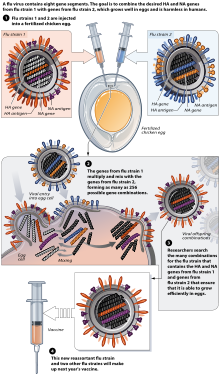
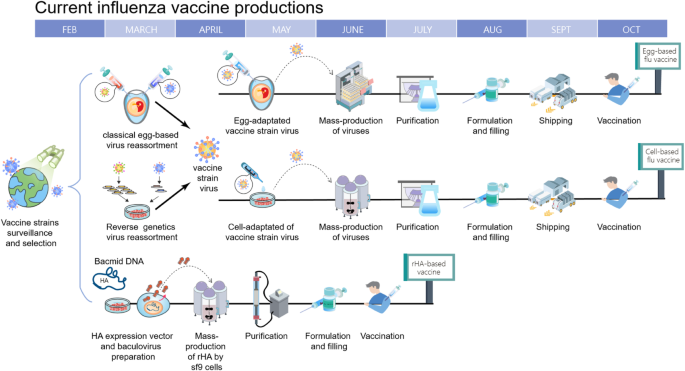
Influenza Vaccine Production and Design The most common method used to produce each years seasonal flu vaccine involves a laborious time-consuming process in which scientists must select vaccine strains months in advance of the upcoming flu season and then grow the selected flu virus strains in chicken eggs. This technique has its limitation in that it requires one fertilized egg per produced vaccine dose. FDA tests and approves all influenza vaccines prior to release and shipment.
Vaccine manufacturers have projected that they will supply 188 to 200 million doses of influenza vaccine for the 2021-2022 season. There are several different manufacturers that use this production technology to make flu vaccines for use in the United States. SNY announced today that its first doses of Fluzone Influenza Vaccine for the 2.
Nowadays influenza vaccines usually consist of either split viruses or subunit influenza antigens. Most inactivated flu vaccines are produced by growing flu viruses in eggs. In particular a trypsin concentration of 5.
For most influenza vaccine production this is performed in nine to twelve-days old fertilized hens eggs. Vaccine bulk manufacture. Flu vaccine is produced by private manufacturers so supply depends on manufacturers.
The vaccine virus is injected into thousands of eggs and the eggs are then incubated for two to three days during which time the virus multiplies. The aim of this study was to evaluate the impact of different inactivation and splitting procedures on influenza vaccine product composition stability and recovery to support transfer of process technology. There is an essential need for the development of next-generation vaccines with a broad range of immunogenicity against antigenically drifted or shifted influenza viruses.
Cell-based refers to how the influenza flu vaccine is made. The flu viruses used in the cell-based vaccines are grown in cultured cells of mammalian origin instead of in hens eggs. In this study we were able to demonstrate that trypsin also interferes with pathogen defence mechanisms of host cells.
This method needs millions of eggs requiring orders to be placed about a year prior to production. Experience in use of adjuvants. Ensuring Alignment and Sustainability Second WHO Consultation on Global Action Plan for Influenza Vaccines GAP-II Robert W Malone MD MS Consulting Vaccines and Biologicals Bench to Bedside.
Trypsin is commonly used in MadinDarby canine kidney MDCK cell culture-based influenza vaccine production to facilitate virus infection by proteolytic activation of viral haemagglutinin which enables multi-cycle replication. While research towards developing universal influenza vaccines is ongoing the current strategy for vaccine supply in a pandemic relies on seasonal influenza vaccine production to be switched over to pandemic vaccines. This production method requires large numbers of chicken eggs to produce vaccine and may take longer than other production methods.
Increased development of mammalian cell lines for vaccine production. Once manufacturing activities begin egg-derived vaccines can take anywhere between 6 and 9 months to be produced before they are ready for distribution2. Rapid development of reverse genetics technologies for generation of vaccine viruses.
Expression vectors are used to make large amounts of antigen to be used as a vaccine. Four split and two whole inactivated virus WIV influenza vaccine bulks were produced and c. Fortunately many next-generation influenza vaccines are currently in development utilizing an array of innovative techniques to shorten production time and increase the breadth of protection.
Production of influenza WIV and split vaccines Six different influenza vaccine batches of bulk vaccine product were produced starting from one batch of clarified allantoic fluid. Traditionally influenza vaccines are produced through the use of chicken eggs from qualified facilities in which viruses are grown and harvested. Vaccines will be an important element in mitigating the impact of an influenza pandemic.
Flu vaccine is produced by private manufacturers so supply depends on manufacturers. The main characteristics of the performed production processes are summarized in Table 1 whereas the respective accompanying process flowcharts are presented in Fig 1. This review summarizes the production methods of current vaccines recent advances that have been made in influenza vaccine research and highlights potential challenges that are yet to be overcome.
Instead of using a virus or bacterium A single gene usually a surface glycoprotein of the virus can be expressed in a foreign host by Cloning. These differences also have an impact on the immunogenicity of the vaccine. Since 1990 there have been significant new developments in methods of influenza vaccine production.
Bird Flu Vaccine Production Processes Infographic
Better Influenza Vaccines An Industry Perspective Journal Of Biomedical Science Full Text

Current And Next Generation Influenza Vaccines Formulation And Production Strategies Sciencedirect

Making The Flu Vaccine A Race Against The Clock Sanofi

5 The Yearly Influenza Vaccine Production Timeline Begins In January Download Scientific Diagram

A Tale Of Two Mutations Beginning To Understand The Problems With Egg Based Influenza Vaccines Sciencedirect

Development Of A Universal Influenza Vaccine The Journal Of Immunology

A Cell Based Backup To Speed Up Pandemic Influenza Vaccine Production Trends In Microbiology

Timeline Of Current Influenza Vaccine Production Methods Schematic Download Scientific Diagram

Hassle Free Influenza Vaccine Close To Reality

Weathering The Influenza Vaccine Crisis Nejm

Flu Vaccine Production Process For The Southern Hemisphere Youtube

Egg Based Pilot Influenza Vaccine Production Process Download Scientific Diagram
Cilian Ag The Ciliate Vantage For Biopharmaceuticals

Pandemic Influenza Vaccine Manufacturing Process And Timeline

Egg Based Influenza Vaccines Esco Vaccixcell

Timeline Of Current Influenza Vaccine Production Methods Schematic Download Scientific Diagram